Mu Lab
Under the Scope – Spring 2021
… Continue Reading
Looking for Degenerative Myelopathy or Other Canine DNA testing?
Our neighbors at Dr. Gary Johnson’s DNA Lab can help! They offer DNA testing for DM and several … Continue Reading
FDA Alert: Certain Lots of Sportmix Pet Food Recalled for Potentially Fatal Levels of Aflatoxin
The U.S. Food and Drug Administration, in cooperation with the Missouri Department of Agriculture, … Continue Reading
Now Available: New 2020-2021 List of Services and Fees
Download now. … Continue Reading
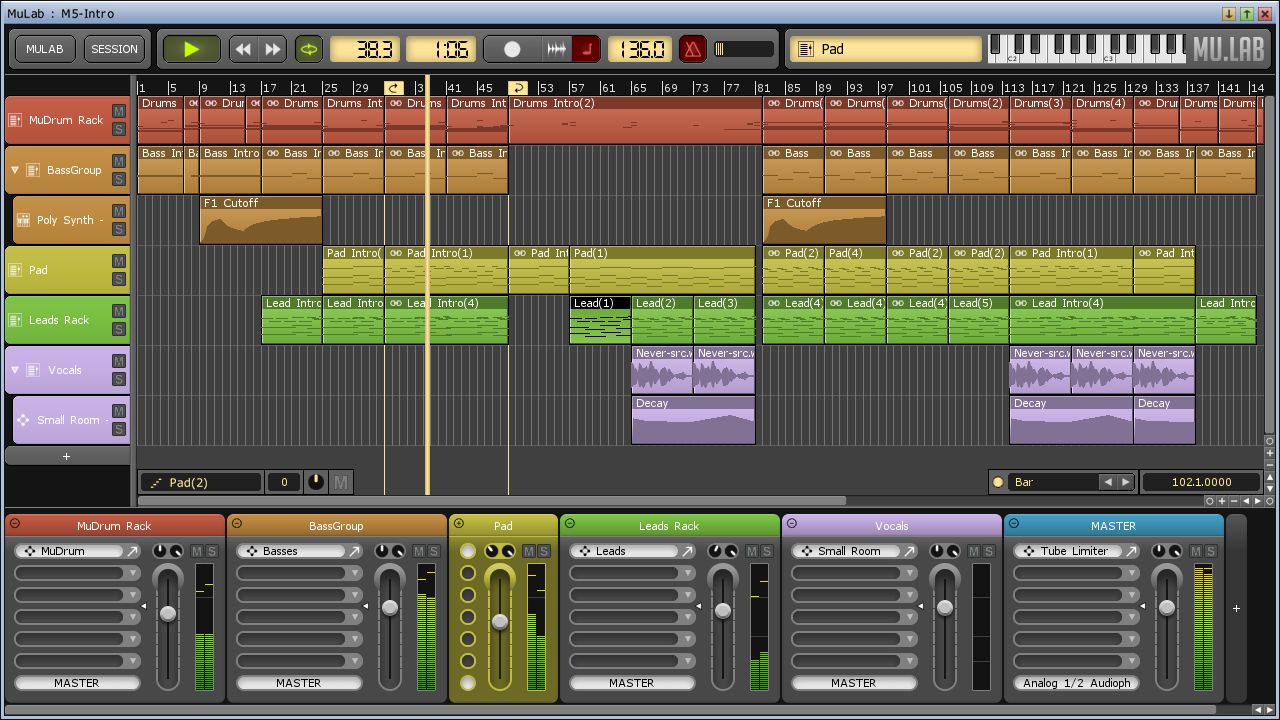
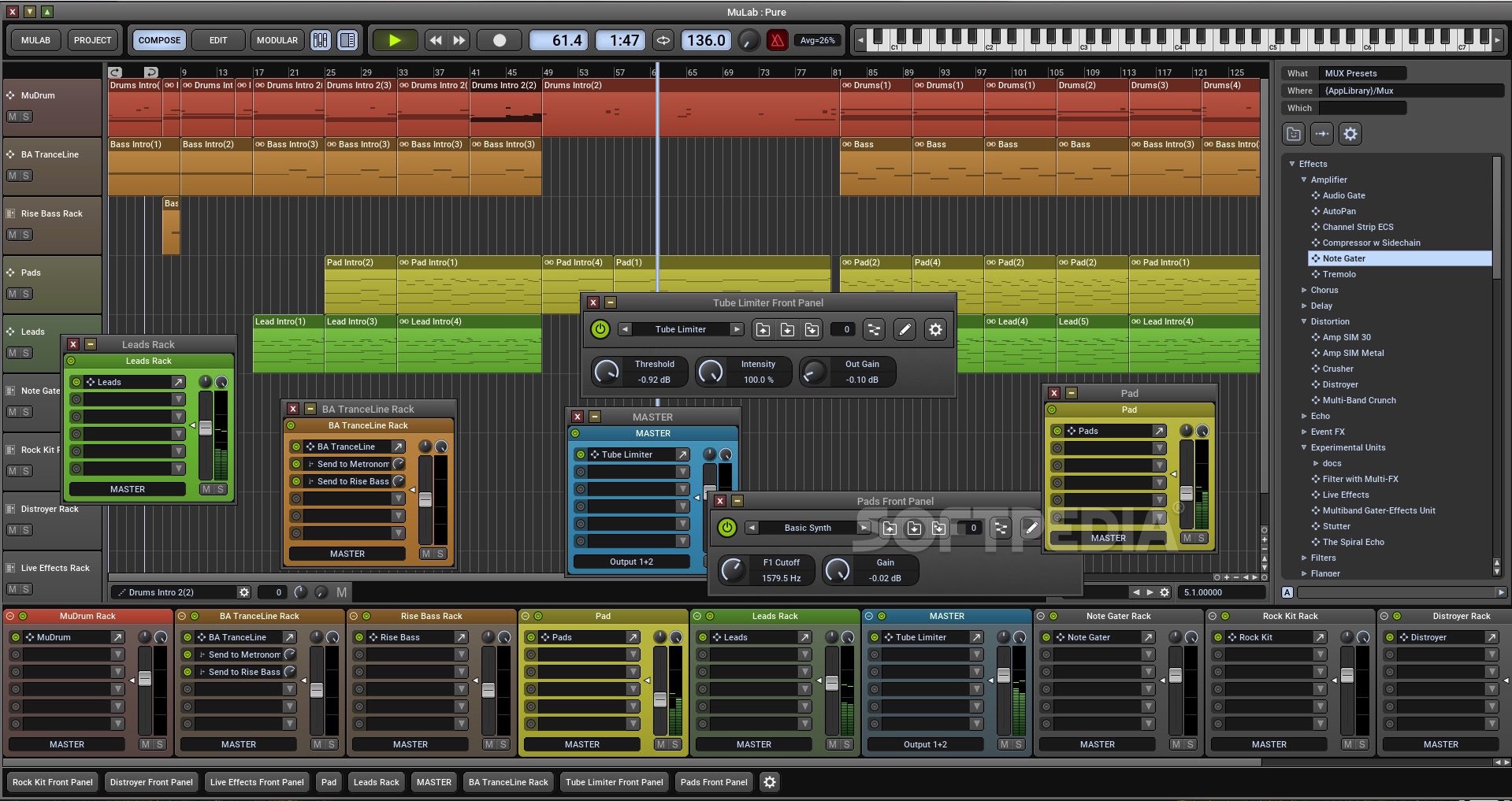
MuLab is a powerful tool designed to transform your computer into a fully capable music production studio. The program allows you to work with various music tracks and instrument samples in order.
NOTE: Experienced investigators looking for specific information can proceed to:
This section provides an introduction to the analysis of data obtained from using small extracellular electrodes to record neural activity. We begin this section by assuming that the electrophysiology has already been done -- i.e., you've manufactured or purchased your electrodes (and by some unknown and miraculous set of circumstances they work), and you've gotten a nervous system to cooperate by permitting you to record neural activity without introducing too much noise. What now?
- MuLab is an inspiring music production studio for Mac OS X and Windows featuring an integrated top-quality modular synth and effect engine. MuLab is an easy, effective and rock-solid tool designed to create, record, edit and finalize your music.
- MuLab is a top-quality sound and music production system for Mac OSX and Windows, transforming your computer into an inspiring modular studio. MuLab is an easy, effective and rock-solid tool designed to create, record, and finalize Your Music!
Basically what you have after your electrophysiological recordings is 'raw' data, which consists of a large set of waveforms from each electrode. Since each electrode samples an area -- not necessarily small with respect to the size of neurons -- the waveforms from any one electrode may consist of spikes (i.e., 'action potentials') from numerous cells. (See Figure 1.) What you'd really like to do (if you're fussy, like us) is to distinguish between spikes of different neurons. In other words, it's time to do some sorting. (Intracellular electrodes, on the other hand, record the membrane potential of a single cell and thus have no need for sorting procedures. But for various reasons, intracellular recording in vivo from mammalian nervous systems is extremely difficult and won't be discussed here.)
Figure 1
It is generally accepted that the action potentials of most neurons have basically the same shape. However, the action potential of different cells, as seen by the recording electrode, will be distinguishable due to differences in the size and shape of the neurons as well as their varying orientation with respect to the electrode. This means that different neurons will produce at least slightly different recorded waveforms when they fire an action potential. Unfortunately (there's always an unfortunately somewhere), since we're recording very small signals, noise can be a problem and can make the sorting process rather difficult -- noise introduces variability, which means that even waveforms from the same cell are not identical each time they're recorded. We won't explain the sorting procedure here in any detail, but basically it involves finding some sort of measurements, or variables, that can help you distinguish between different waveforms. For instance, you can do principal component analysis on the waveforms, or perhaps something simpler, like finding peak-to-peak values. After you have identified these variables, you plot each waveform using the variables as axes; what you hope (and sometimes it actually happens!) is to see clusters of points, representing waveforms that are similar with respect to the variables. You are then justified in taking a cluster as representing a single cell, if the cluster is obviously distinct from the other waveforms in the sample. Thus, each waveform in the cluster is labeled as coming from the same cell.
Now what you have is some waveforms grouped into bunches based on their shape, and you also know at what time each waveform occurred. You don't really care much about the waveform shape in subsequent analysis; that was important only in distinguishing between the spikes of different cells (and between a spike and noise). So what you do next is simply plot the spikes of each neuron as a function of time, thus creating what's known in the business as spike trains. An example is shown in Figure 2, which consists of a segment of two simultaneously recorded spike trains, labeled NEURON 1 and NEURON 2. The vertical bars represent a spike. Such plots are also called rasterplots.
Figure 2
Rasterplots are important in giving you a detailed look at the data, but there are other ways to more compactly present your results. One very popular method is to make histograms . Before describing histograms, however, we need to make a slight digression to discuss two basic types of electrophysiological experiments, since data is analyzed a little bit differently depending on which type was performed.
In some experiments, neurons are recorded without stimulation, i.e., their spontaneous activity is recorded; in these experiments, you are basically recording neural activity while the nervous system is 'resting'. In other experiments, you may wish to record while the nervous system is doing something, and in such cases you generally provide some sort of stimulation which you can control (the important variables to control are things like the intensity of the stimulation and its time duration). In these experiments, the neurons you are recording from are 'activated' or stimulated, if you use a stimulus which will excite the neuron (in other words, if you use a stimulus to which the neuron is tuned). For cells in the visual system, for example, presentation of a bar or spot of light in the appropriate part of the animal's visual field will excite the cell, elevating its firing rate.
Now to describe a histogram: it's just a plot of the binned data as a function of time. For spontaneous recording, you simply break the long spike train into small time segments, which are called bins, and add the spikes in each bin. Figure 3 shows an example of this process. The ellipsis (three dots) on each side indicates that the figure has been abbreviated for display purposes.
Figure 3
For stimulated recordings, something a bit different happens. In most cases, the stimulus in such recordings is repeated numerous times, producing identical 'trials' in the experiment. This is done because there is quite a bit of variability in most neurons' responses, even to the same stimulus; in other words, even when given identical stimuli a neuron's spike train can be vastly different across trials. You therefore need to get a sufficiently large sample of a neuron's response in order to make statistical inferences about its average response to that stimulus. In order to make a histogram under these circumstances, you cut out a segment of the spike train corresponding to each trial, and line them up based on the time of the 'stimulus marker' (which is a time stamp that you perspicaciously placed during your recording to mark when you began delivering a certain stimulus). Figure 4 shows an example of this process. The time that the stimulus ended is also known to you, of course, and this influences your choice of the length of a trial segment. When so constructed, the histogram is called a peri-stimulus time histogram or PSTH for short. (If you only analyze the data following the stimulus, the histogram is called a post-stimulus time histogram -- but this distinction isn't really important. Besides, the acronym is the same in both cases.)
Figure 4
So you now know about rasterplots and histograms, which are basically ways to display single unit data. Is there other information that can extracted from single unit data? There is, in fact, and for experimenters who are so impoverished that they can only record from one cell at a time, it's the only sort of information they can squeeze out of their data. (Note: Some experimenters lump all the data from their single electrode recordings into 'one neuron', which is actually a combination of the neurons which that electrode sampled. In other words, they fail to sort their data. Such 'single unit' data is occasionally referred to as dirty data.)
We won't spend much time discussing some of the single unit analytical methods, but some of the more important can be briefly described:
One of the simplest things to do is to normalize the PSTH; you do this by dividing the bin counts by the binsize (in whatever units of time you wish to use), and by the number of trials if you used a repeated stimulus. This results in a display of the average firing rates of the neurons -- i.e., a graph of the number of spikes per unit time over the course of the experiment or stimulus response.
You can also make a bar plot of the interspike intervals, which are simply the time between spikes of the spike train. For example, if there were spikes at a time of 240 milliseconds (with respect to some reference marker), 249 ms, and 262 ms, then you would increment by one count the bin containing 9 ms intervals and the bin containing 13 ms intervals. Note that for a spike train of n spikes, you would have n-1 interval values. Different types of spike trains will have different distributions of interspike intervals.
One further analytical tool is the autocorrelogram (which is related to the crosscorrelogram described below); this function lets you discern the fine time structure, if any, in the spike train of a single neuron.
But there are much more interesting kinds of data analysis that you can do with multiple unit data...
Next: Crosscorrelation
Mulabandhasana
Mulab Download
